Answer 1 of 4. Cellulose is an example of a polymer formed by beta-glucose.
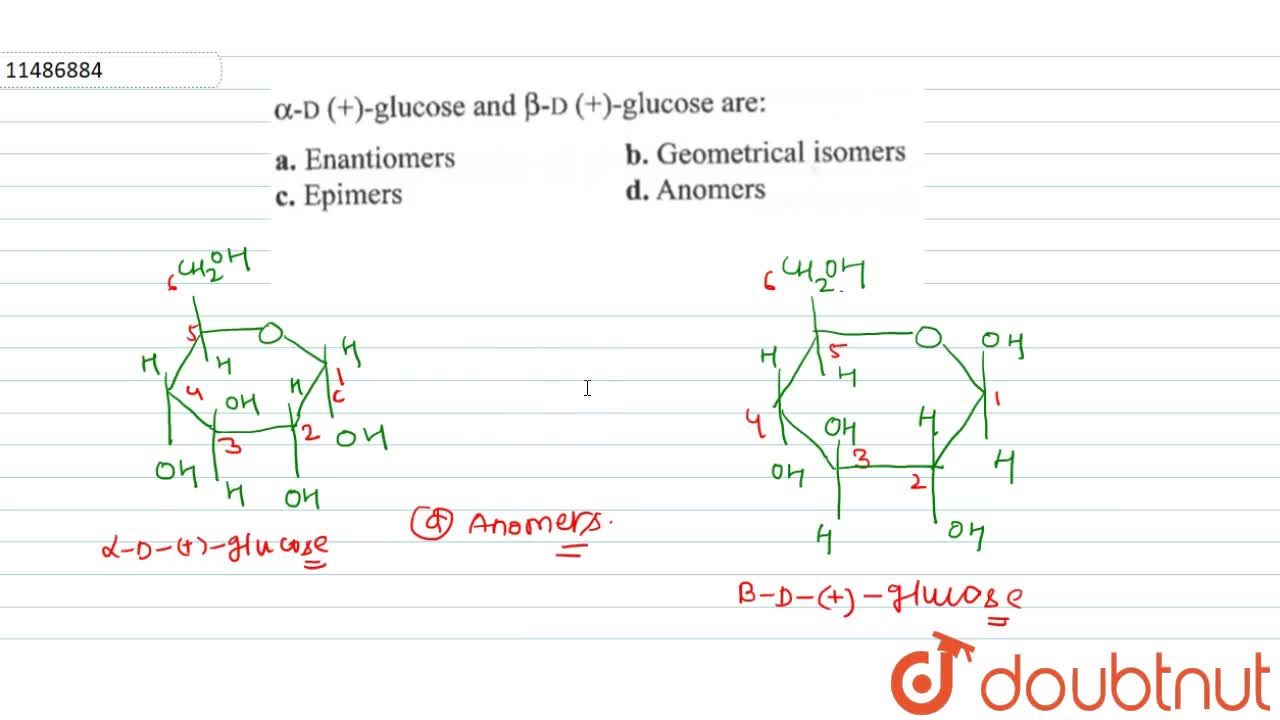
Alpha D Glucose And Beta D Glucose Are
Anomers of glucose arc cyclic diastereomers differ in configuration are C-1 existing in two forms α - and β -respectively.

. α -D-glucose and β -D-glucose is example of anomers. Chemistry questions and answers. State whether the following statements are True or False.
Alpha D and beta D glucose are not enantiomers. These are ALPHA BETA ANOMERS. An Anomer of a saccharide only differs in its structure at the anomeric carbon.
TrueFalse If you are seeing this message that means JavaScript has been disabled on your browser please enable. Deve you coz our stereo buy summers so moving towards option B we have to show the condensation off to Lucas molecule from a dissection right along with Al Fahad like acidic linkage. Alpha D and beta D glucose are not enantiomers.
These are ALPHA BETA ANOMERS. They differ only in the direction that -H and -OH groups point on carbon 1 See the jmol images below. Alpha D glucose can be written as α-D-glucose whereas bet D glucose can be represented as β-D-glucose.
These are mirror images and superimposable. Such isomers are referred as anomers. These are ALPHA BETA ANOMERS.
Click hereto get an answer to your question alpha - D - - glucose and beta - D - - glucose are. Option C is correct. The formation of hemiacetal and hemiketal generates a new assymetric carbon which is called as anomeric carbon.
This means that they differ in the configuration at C1 ie carbon one atom in the case of aldoses and C2 in the case of Ketoses. When alpha-glucose molecules are joined chemically to form a polymer starch is formed. The term anomers of glucose refers to isomers of glucose that differ in configuration at carbon one C-1.
Anomeric carbon being the functional group of the carbohydrate which is usually the carboxyl group attached to it. Epimeres differ at only one chiral center not the anomeric carbon. The D-glucose can exist in two forms alpha-D-glucose and beta-D-glucose.
Anomers are capable of interconverting in solution. As alpha and beta glucose do not meet the criterion they are not enantiomers. The generation of new assymetric center at carbonyl compound results in 2 isomeric.
α-D-glucose and β-D-glucose are stereoisomers - they differ in the 3-dimensional configuration of atomsgroups at one or more positions. Anomer An anomer is actually an epimer also a cyclic saccharide that differs in configuration particularly at the acetal or hemiacetal carbon refer to the image below to differentiate between acetal and hemiacetal carbons. What is the difference between cellulose and cellulase.
Lets talk about alpha beta glucose they are ANOMERS. A-D Glucose and B-D Glucose are 1 Anomers 2 Epimers 3 Enantiomers 4 All of these Solve Study Textbooks Guides Join Login. Alpha-D-glucose and beta-D-glucose are examples of __________.
α D -glucose and β D -glucose are those diastereomers that differ in configuration at C- 1 atom. Methyl α -D-glucoside is actually a. Because D L forms are enantiomers - which are mirror images that can not be superimpose.
More specifically they are a class of stereoisomer called an anomer. Alpha-D-glucose and beta-D-glucose are examples of __________. Click hereto get an answer to your question 8.
Alpha D and beta D glucose are not enantiomers. The formation of hemiacetal and hemiketal generates a new assymetric carbon which is called as anomeric carbon. A enantiomers B anomers C furanoses D polysaccharides Which structure best describes the structure of tyrosine at.
Alpha-D-glucose and beta-D-glucose are enantiomers. α -D-glucose and β -D-glucose are anomers. These are ALPHA BETA ANOMERS.
This means that they differ in the configuration at C1 ie carbon one atom in the case of aldoses and C2 in the case of Ketoses. This means that they differ in the configuration at C1 ie carbon one atom in the case of aldoses and C2 in the case of Ketoses. Worse V arrest al for D glucose on dhe beater.
BThe tautomers are known as the isomers which differ only in the position of the protons and electrons. Thus they are not enantiomers. Because D L forms are enantiomers - which are mirror images that can not be superimpose.
Now the alpha - D - glucose and beta - D - glucose are non-superimposable as they do differ in the position of the hydroxyl group at carbon 1 but they are not the exact mirror image of each other. Alpha D and beta D glucose are not enantiomers. Lets talk about alpha beta glucose they are ANOMERS.
Are alpha-D-glucose and beta-D-glucose enantiomers. Both αDglucose and βDglucose have the same specific rotation. So the answer for this option is no Alfa blue coz and beater glucose are not in action.
Enantiomers are the non-superimposable mirror images of the isomeric structures of same molecule. This means that they differ in the configuration at C1 ie carbon one atom in the case of aldoses and C2 in the case of Ketoses.
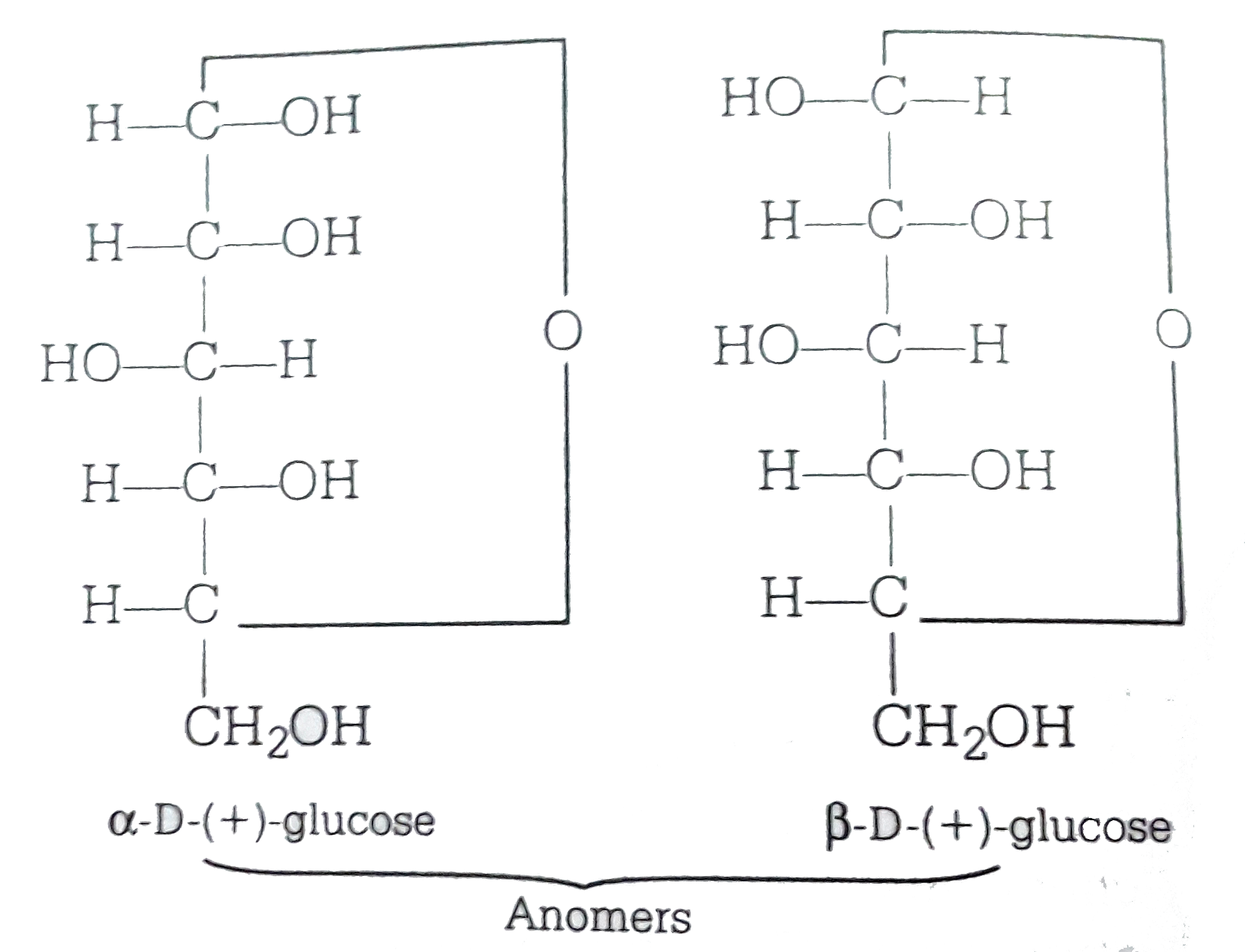
Alpha D Glucose And Beta D Glucose Are

0 Comments